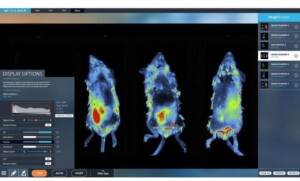

IN VIVO 3D optical tomography - NOW ACCESSIBLE TO EVERYONE.
Description
The Newton 7.0 is an innovative optical bioluminescence, fluorescence, and 3D tomographic imaging system designed with the user in mind.
It is ideal for in vivo, ex vivo and in vitro imaging applications, allowing for simultaneous imaging of multiple animals or samples at a time. It’s advanced features and software are easy to navigate and optimized for a multi-user interface. Furthermore, the intuitive workflow and advanced system sensitivity facilitates time-saving signal acquisition for longitudinal studies.
This highly sensitive optical imaging system is dedicated to pre-clinical imaging of small animals in vivo, and may also be used on a variety of in vitro and ex vivo samples. It combines the best optics and animal handling features for optimum scientific images and results. The Newton 7.0 systems are capable of bioluminescence, fluorescence as well as 3D tomographic imaging. The system is:
- User-friendly
- Does not require any radiation to acquire images
- Is non-invasive, allowing for longitudinal studies
- Allows for up to 5 mice or 3 rats to be imaged simultaneously
Testimonial
Customer from University Hospital Basel | Brain Tumor Immunotherapy:
„The high sensitivity of the Newton enables us to detect the intracranial tumors more precisely and much earlier, which improved our experimental treatment regimens significantly“
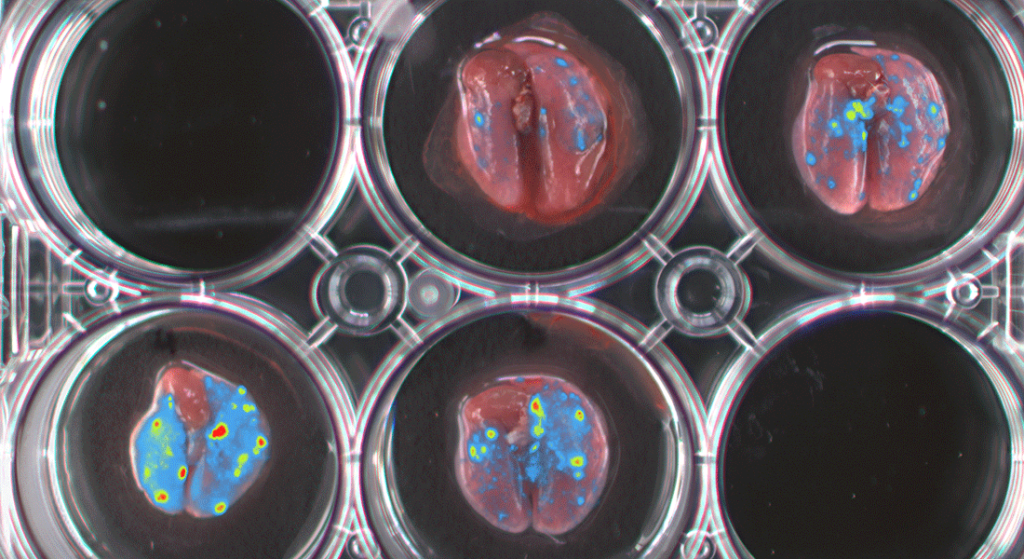
Lungs presenting micro & macro metastases from MDA-MB-231 expressing GFP-Luciferase – Image property of University Basel
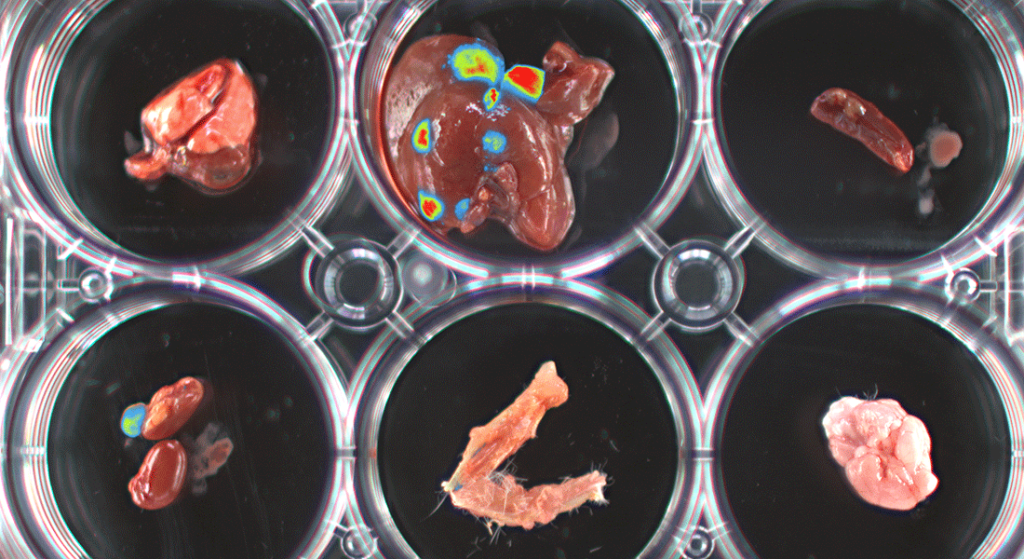
Image property of University Basel
WEBINAR
Vilber’s Newton 7.0 FT Series – in vivo 3D Optical Tomography now accessible to everyone
This free educational webinar hosted by Scintica Instrumentation introduced the brand new pre-clinical imaging system from Vilber, the Newton 7.0 FT Series. This new range of in-vivo imaging systems offer traditional 2D Bioluminescence and Fluorescence imaging, integrating an innovative 3D Optical Tomography technique to provide even further details in terms of signal structure and localization.
Click here for the video (60min.)

Powerful Fluorescence Excitation
The Newton 7.0 offers 8 excitation channels in the visible RGB and near infrared spectrums. The very tight LED spectrum is additionally constrained with a very narrow excitation filter; these excitation sources are categorized as a Laser Class II due to their intense power. Movement of the excitation source over the entire FOV ensures consistent and reproducible results over the course of a longitudinal study.
Full Spectrum Tunability
8 excitation channels and 8 emission filters are available to cover the complete spectrum from Blue to infra-red.
Narrow bandpass filters are used for both excitation and emission to reduce cross talk between dies, allowing for up to 3 dyes to be imaged simultaneously.
Macro-imaging to large throughput studies
Vilber’s intelligent darkroom architecture allows for fully automated movement of the camera (Z-axis) and animal pad (X/Y axis) to move through both the macro imaging FOV (6x6cm) to the full FOV (20x20cm) for imaging up to 5 mice.
Spectral Unmixing
Spectral unmixing is possible for both bioluminescence and fluorescence imaging when different luciferase enzymes or fluorescent dies are used.
The includes algorithms to remove crosstalk between the different signals, allowing for each channel to contain signal from only one reporter.
3D Optical Tomography
An integrated 3D tomography module allows both bioluminescence and fluorescence signals to be reconstructed in 3D and overlaid within a topographical model of the imaging subject.
For better understanding of anatomical and deeper tissue structures, the digital organ library allows for superimposition of the mouse organs and bones onto the topographical model.
State-or-the-art camera technology
- Scientific grade 16-bit CCD
- -90°C delta cooling
- f/0.7 aperture
- 10 megapixel image resolution
- 4.8 Optical Density
The advanced camera and optics provide increased sensitivity to either bioluminescence or fluorescence signals, with a very low signal to noise ratio. The high optical density allows for samples with both very low and high signals to be imaged without saturation, allowing for quantifiable results.
Imaging Modes – Fluorescence
Easily detect fluorescent molecules and reporters at the picogram level in the tissue of interest using Vilber’s dynamic range of excitation emission wavelengths.
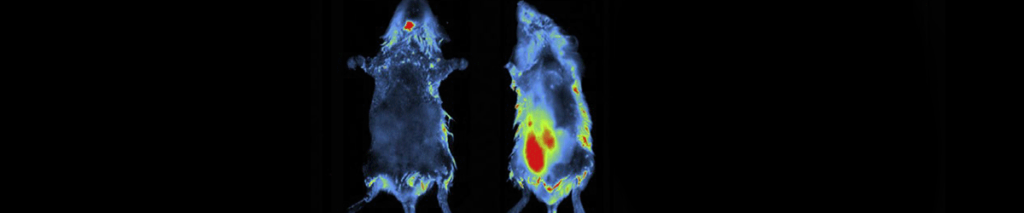
Imaging Modes – Fluorescence
For deeper tissue penetration and reduction of autofluorescence background, infrared (IF) and near-infrared (NIR) molecules can also be imaged with Vilber.
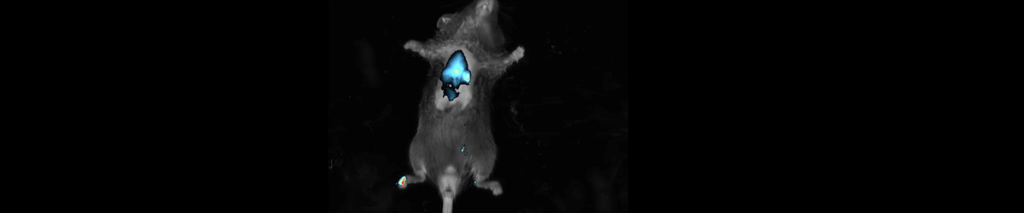
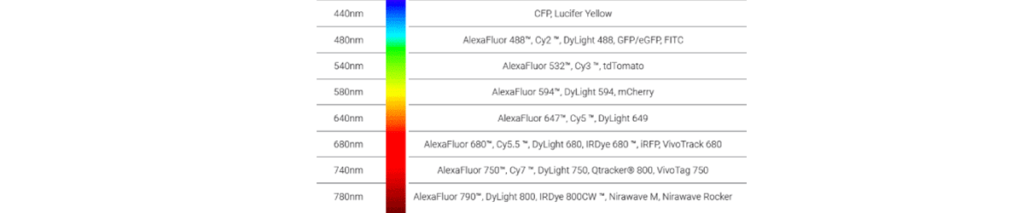
The below dyes can be used with the Newton 7.0 systems:
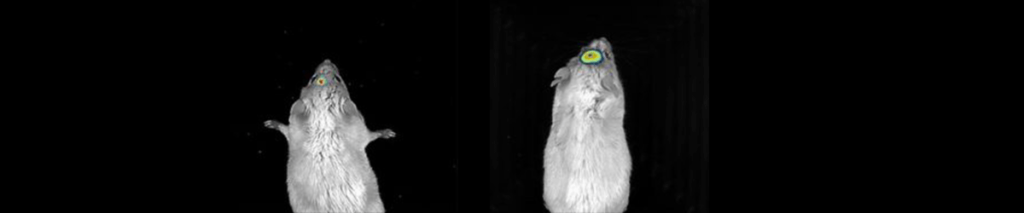
Imaging Modes – Bioluminescence
Bioluminescence imaging can be used to detect luciferase-tagged molecules at the femtogram level.
Imaging Modes – Multispectral Imaging
Multispectral in vivo imaging is possible by using different luciferase enzyme/substrate pairs or by using different fluorescent dyes.
Signals can be overlaid within the same image, up to 3 reporters can be imaged simultaneously.
Imaging Modes – Longitudinal Imaging
Images acquired at different time points can be arranged to form a longitudinal image sequence. For example, a time series could be constructed from images acquired on different days following an experimental treatment.
The software then compares the image data throughout the experimental treatment.
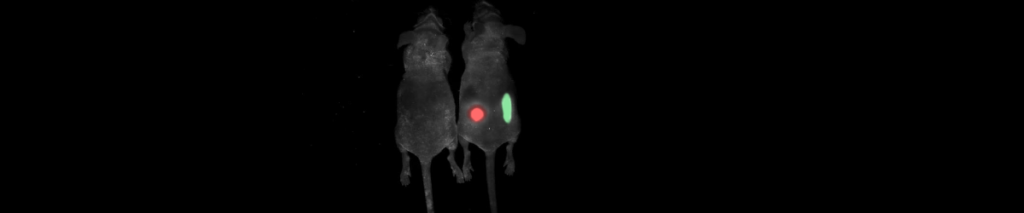
Applications – Arthritis
Inflammatory responses can be assessed using non-invasive fluorescence biomarker imaging in a preclinical model of rheumatoid arthritis.
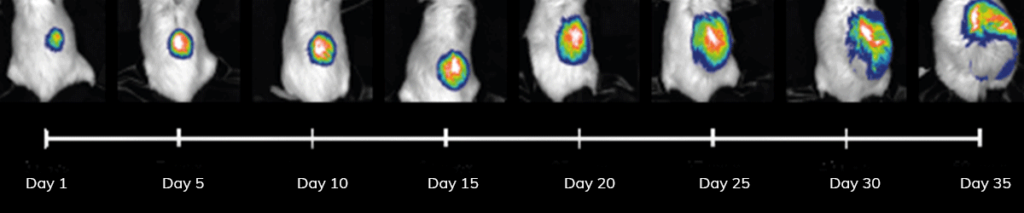
Applications – Tumor Monitoring
Tumor progression can be monitored after establishment of orthotopic tumors in mice. The mouse brain was injected with 10,000 (fig.left ) and 50,000 (fig.right ) cancer cells expressing luciferase.
After a 6 weeks, tumors were formed, where signal was dependent on tumor cell injection quantity.
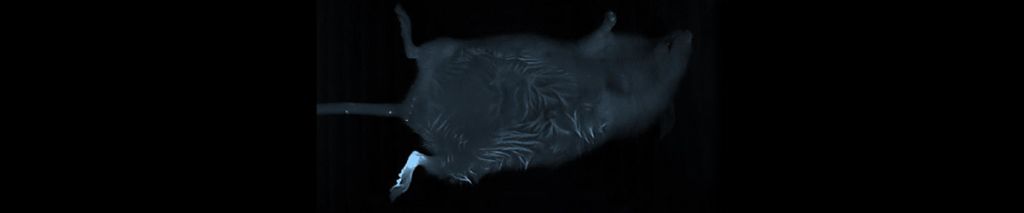
Applications – Biodistribution
Using the Vilber imaging system, researchers investigated the biodistribution of doxorubicin hydrochloride-loaded nanogels in rats (Sprague–Dawley, 220–250 g), click here for the link to the article abstract. The fluorescent signal of DOX was detected and monitored over eight hours, seen right.
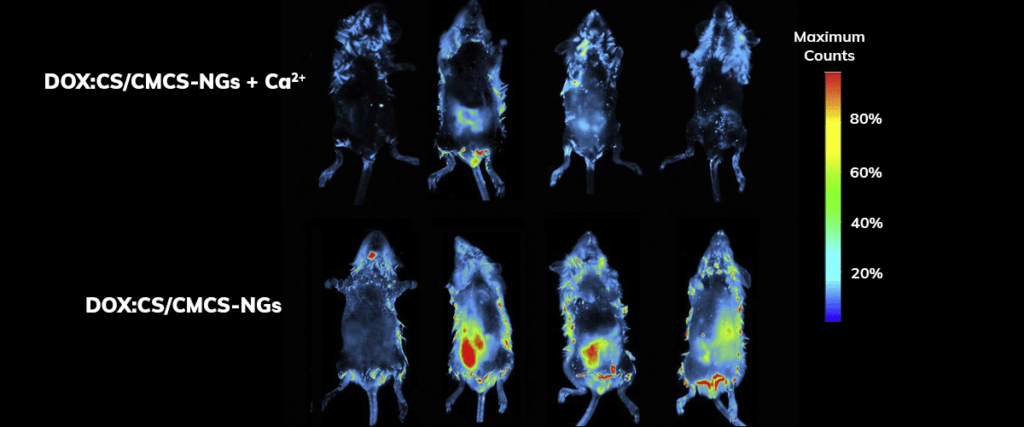
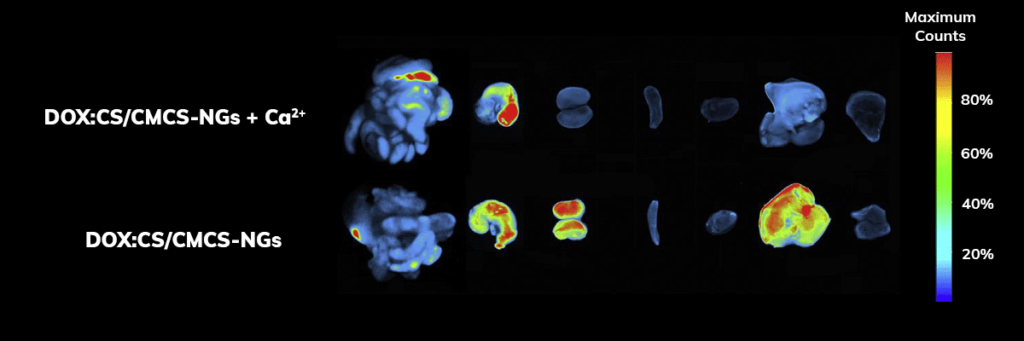
Applications – Biodistribution
The major organs were dissected 10 h after oral administration and observed ex vivo.
Signal quantification demonstrated that organs harvested from rats treated with doxorubicin hydrochloride-loaded nanogels group exhibited significantly higher retention of doxorubicin hydrochloride compared to rats treated with doxorubicin hydrochloride alone.
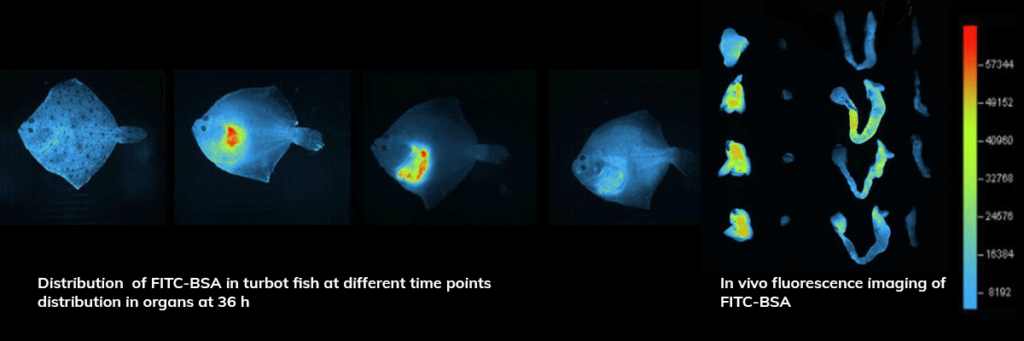
Applications – Biodistribution
In vivo fluorescence imaging of FITC-BSA nanoparticle in the Turbot fish.
After 36 h, the heart and liver were dissected and visualized nanoparticle retention was identified.
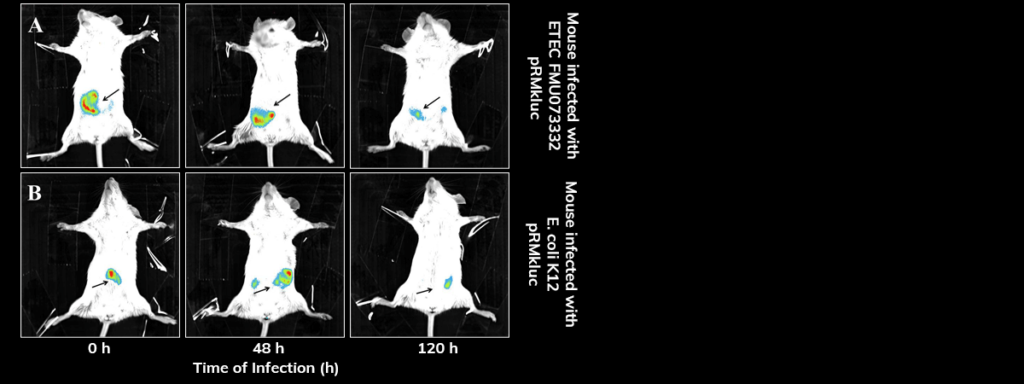
Applications – Gastrointestinal Pathologies
Bioluminescent enterotoxic E. coli (ETEC) is tracked through the mouse intestine, demonstrating the colonization dynamics across the GI tract. Click here for the link to the article abstract.
Streptomycin-treated BALB/c mice were inoculated with E. coli bacteria via gavage with pRMkluc-tagged ETEC and pRMkluc-tagged E. coli K-12. Luciferin was administered intraperitoneally prior to imaging.
After inoculation, bioluminescence was localized to the small intestine. 48 hours post-inoculation, the bioluminescent signals indicated bacterial passage through the mouse intestine. Bioluminescent signals were detected in the mouse intestine up to 120 h post-inoculation.
After 120 h of E. coli infection, mouse gastrointestinal tracts were extracted to perform ex vivo imaging. Intestinal tract dissection included the esophagus to rectum.
ETEC were localized in the proximal mouse ileum approximately 6 cm from the cecum, whereas the E. coli K-12 ol signals were identified in the cecum and in the proximal colon.
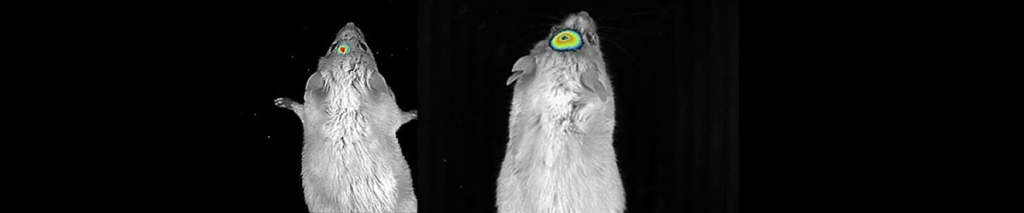
Applications – Infection Tracking
BALB/c mice were intraperitoneally inoculated with E. coli K-12 tagged with pRMkluc or E. coli K-12 tagged with pBR322 (incubated with luciferin). Click here for a link to the article abstract.
After 1 h of infection, bioluminescent signal emission from the animals was captured.
Mice infected with E. coli K-12 harboring pBR322 did not exhibit bioluminescent emission (Fig.B). However, mice infected with E. coli harboring pRMkluc emitted bioluminescent signals detected in the mouse inoculation zone (Fig.A).
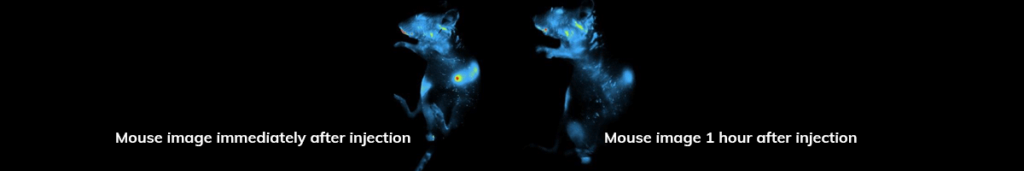
Applications – Toxicology
Screen localization dynamics of fluorescently-tagged drugs. Investigators injected a Cy5.5-tagged drug and tracked it’s spread systemically in the mouse.
NEWTON Models
| BT100 | FT100 | BT500 | FT500 | |
| Camera | DarQ-9 1” Scientific CCD Sensor 2160×2160 (4.6 MP) | DarQ-9 1” Scientific CCD Sensor 2160×2160 (4.6 MP) | DarQ-9 1” Scientific CCD Sensor 2160×2160 (4.6 MP) | DarQ-9 1” Scientific CCD Sensor 2160×2160 (4.6 MP) |
| Cooling | -90°C | -90°C | -90°C | -90°C |
| Lens | f/.070 | f/.070 | f/.070 | f/.070 |
| Bioluminescence | Yes | Yes | Yes | Yes |
| VIS/NIR Fluorescence | upgradeable | 400 > 900nm | upgradeable | 400 > 900nm |
| 3D Optical tomography | Yes | Yes | Yes | Yes |
| Emission Filter-Wheel | 11 positions | 11 positions | 11 positions | 11 positions |
| Field Of View | 12 x 12cm | 12 x 12cm | 6 x 6cm to 20 x 20cm | 6 x 6cm to 20 x 20cm |
| Animal Capacity | 1 or 3 mice | 1 or 3 mice | 5 mice / 3 rats | 5 mice / 3 rats |
| Heated Stage | Yes | Yes | Yes | Yes |
| Anesthesia Station | Optional | Optional | Optional | Optional |
Specifications
| Performance |
Bioluminescence detection : femtogram level |
| Camera & Optics |
Scientific grade CCD camera Motorized V.070 lens: f:0.70 |
| Animal Management |
BIOSTHESIA gas anesthesia module |
| Hardware Capabilities |
Intelligent Darkroom concept |
| Illumination & Filters |
Epi-illumination |
