For in vitro diagnostics use (CE-IVD)
Description
For in-vitro diagnostics use. This product is characterized by the EchoLUTION single-step purification technology, an instant lysis without incubation for swab samples, and a fast lysis step of 10 minutes for stool samples. Together, the overall extraction time is reduced to a minimum and the workflow results in consistent performance.
The product complies with the European In-Vitro Diagnostic Regulation (IVDR) and is CE-marked.
- Convenience and speed: Single-step purification allows complete extraction of 2 x 96 swab samples within 20 minutes. Appropriate for any lab—from low to high throughput and for manual and automated workflows.
- High compatibility: Compatible with nasopharyngeal swabs and oropharyngeal swabs and different types of non-chaotropic and chaotropic resuspension and transport media as well as for stool samples.
- Validation: IVDR-compliant and CE-marked. It can be integrated into diagnostic laboratory routines and ensures high-quality standards.
- Reliable results: With no organic solvents added and by reliable removal of other inhibitors, kit usage results in high-quality viral RNA or DNA for downstream applications like (RT-)PCR.
- Sustainability: Up to 70% less plastic consumption compared to other extraction methods.
The conditioning plate is not included in the kit, as it can be used multiple times. The lysis plate is also not included in the kit.
The extraction workflow: faster and fewer steps
- Only 3 steps needed
- Whole workflow up to 3x faster
The tailored lysis step works without incubation (or a short incubation for stool samples), and the purified viral RNA or DNA is ready-to-use within 20–30 minutes depending on the sample type (swabs in medium, dry swabs, or stool samples).
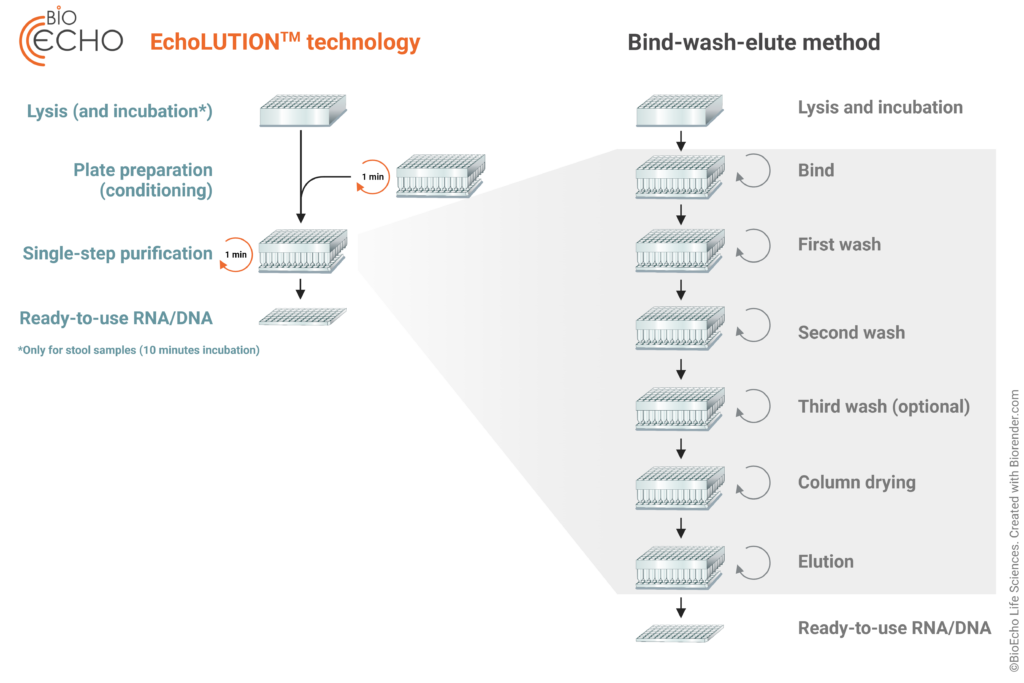
- Lysis (and incubation)
The LyseNtact Buffer New Formula immediately inactivates and lyses the viral particles. For respiratory viruses, no incubation is needed. For enteropathogenic viruses from stool samples, the Lysis Plate is incubated for 10 minutes at 95 °C, to ensure all viral capsides are disrupted. - Single-step purification
Once the lysate is transferred onto the Purification Plate, it is purified in a one-minute centrifugation. The RNA/DNA passes through the purification matrix without further interaction while impurities and cellular debris are held back and removed. - Ready-to-use RNA/DNA
This innovative technology provides RNA/DNA that is ready-to-use for downstream applications.

