An Accurate Automated Cell Counter for cGMP
Description
KEY FEATURES & BENEFITS
- Less than 25 sec to get results
- Cell therapy quality control
- 21 CFR part 11 compliance
- Stem cell. CAR-T cell, CAR-NK cell, Adipose-derived stem cell
- Accurate cell counting and viability
- Automated image analysis
- 4TB extra storage for data backup and recovery
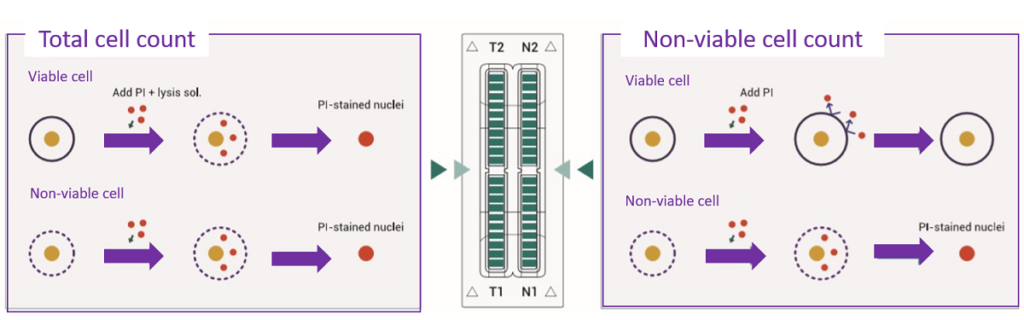
Viability Measurement
PI-Staining Method
After the samples are stained with fluorescent dye, propidium iodide, which intercalates DNA to stain the nucleus of target cells, ADAM™ CellT takes fluorescent images automatically.
The obtained images are processed by image analysis software integrated inside the system.
Data Management
21 CFR Part 11 Compliance
ADAM™ CellT complies 21 CFR part 11 which is a regulation about electronic records and signatures for use in cGMP facilities.
When 21 CFR Part 11 mode is activated, data cannot be modified by any user.
Every action of users is recorded in an audit trail which includes the date, time, and specific details of the action. Electronic signatures based upon biometrics are designed to ensure that they cannot be reused by, or assigned to, anyone else.
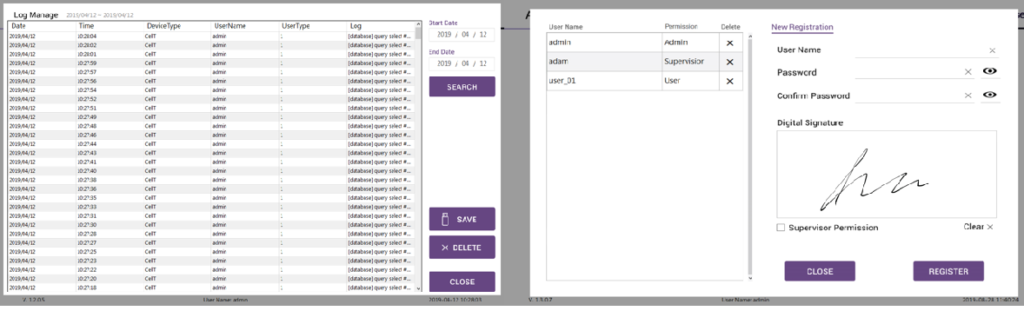
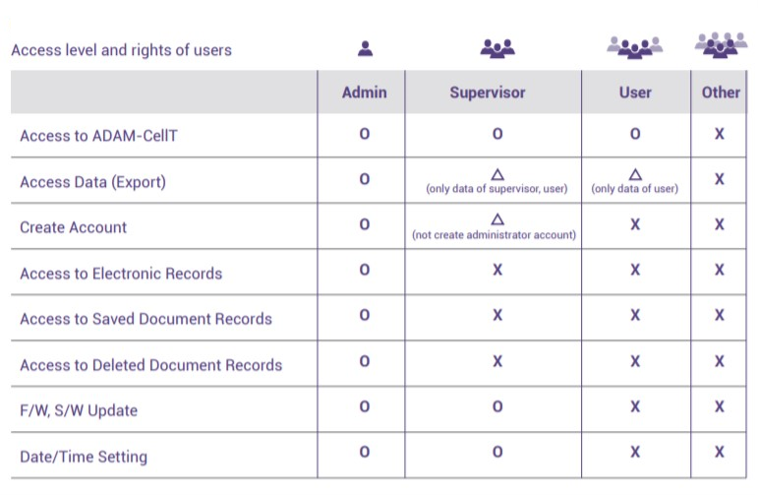
User Management
Access level & rights of users
21 CFR Part 11 consists of limiting system access to authorized individuals. Users are able to control access within software. It is designated to separate 3 different groups – Admin, Supervisor and User.
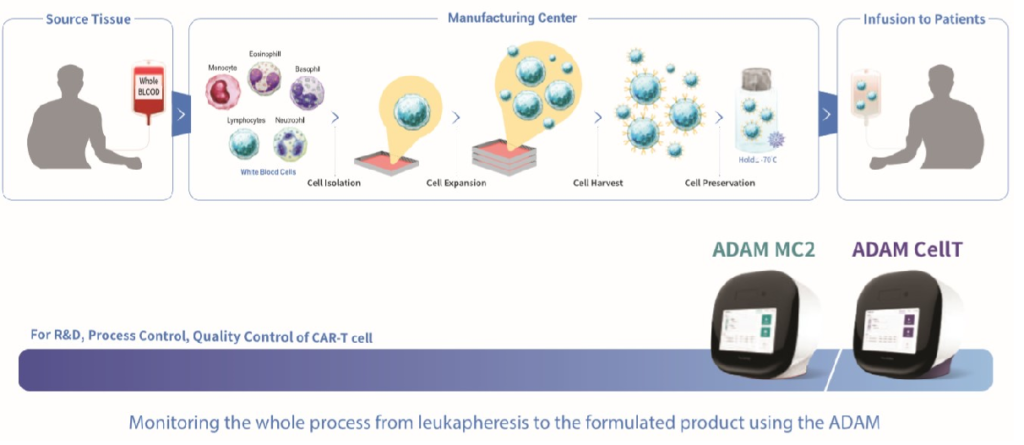
All steps of producing CAR-T cell
QC Platform for producing CAR-T cell
It is easy to monitor all different steps of the purification, expansion, and formulation of CAR-T cells using the ADAM™ to ensure precise and reliable results. It can be used for cGMP, process control and quality control of CAR-T cell.
Data Analysis
Performance test from isolated T-cells
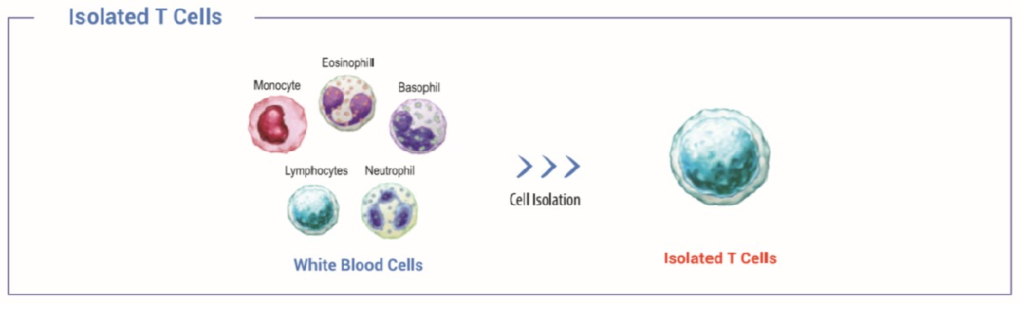
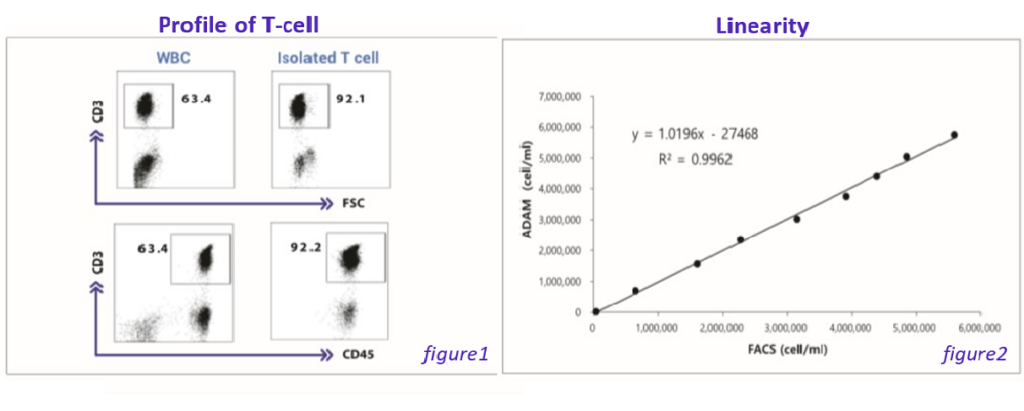
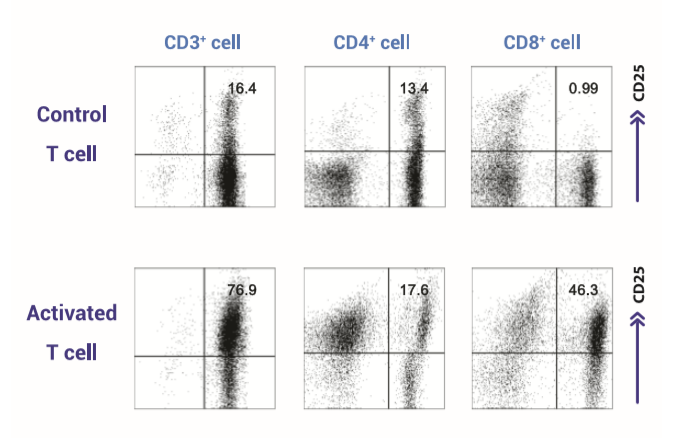
Figure 1 indicates the profiles of T cells that was used for performance evaluation. Flow cytometric analysis of CD3 expression on unsorted or sorted human peripheral blood lymphocytes.
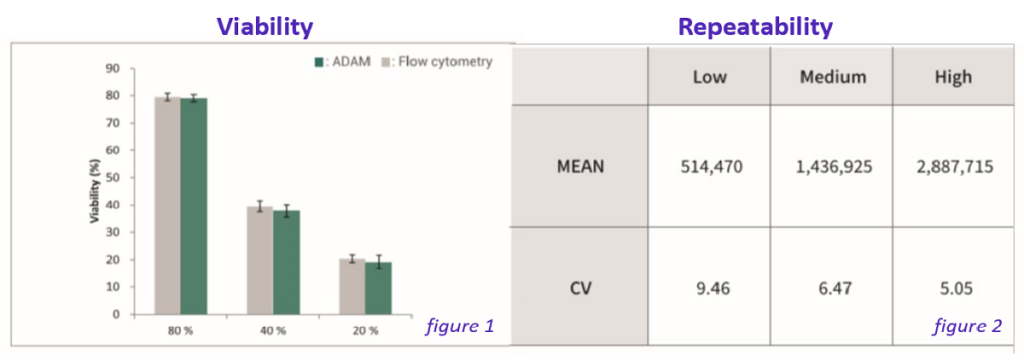
Figure 2 indicates linearity showing comparison between flow cytometry and ADAM™ in Isolated T cells.
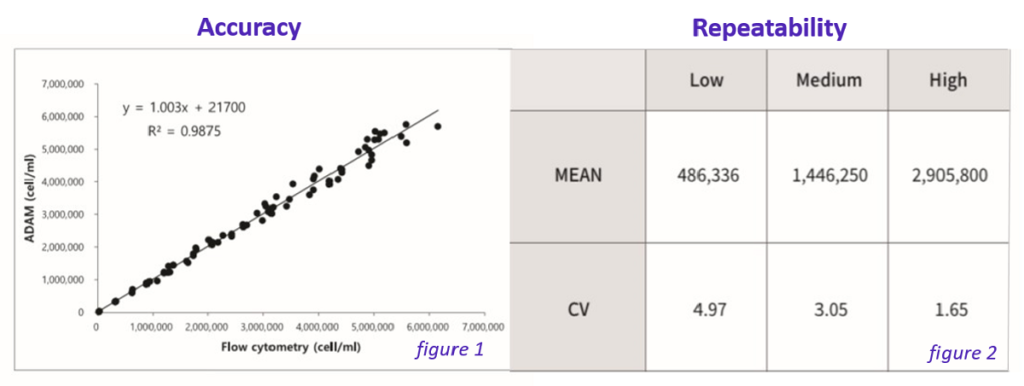
Figure 1 shows accuracy of ADAM™-CellT which is a a correlation of Tcell-counting between flow cytometry and ADAM™ in Isolated T cells.
Figure 2 shows repeatability when the concentration of of isolated T cells is low, medium and high.
Data Analysis
Performance Test from activated T cells
Specifications
| Measuring range |
5 x 104 ~ 2 x 107 cells/mL |
| Optimal range |
4 x 105 ~ 1 x 107 cells/mL |
| Analysis time |
Fast mode: 10 sec/test Real Cell size mode: 30 sec/test |
| Focus |
Auto-focusing |
| Objective lens |
4x |
| Size (L x W x H) |
227x276x270mm |
| Weight |
7 kg |
Accessories
Size: 1 Kit | 200 slides
Staining method - Propidium Iodide (PI)