ADAM-CellT is an automated cell counter that is available in cGMP production environment and complies with 21 CFR part 11.
Description
ADAM-CellT is a new standard of automated fluorescence cell counter. ADAM stands for Advanced Detection and Accurate Measurement. ADAM utilizes sensitive fluorescence dye staining, LED optics and CMOS detection technologies to make the cell analysis more accurate and reliable.
It measures the number of total cells, viable cells, non-viable cells and shows viability results. Combined with a disposable microfluidic chip, the operation is now extremely simple, easy, and cost-effective.
It takes only 25 seconds for ADAM-CellT to present results. ADAM-CellT has an easy-to-use built-in tablet and complies with 21 CFR part 11. When 21 CFR part 11 mode is activated, data cannot be modified by any user. Every action is recorded in an audit trail including the date, time and specific details of the action.
KEY FEATURES & BENEFITS
- Compliance with 21 CFR part 11
- Automated image analysis
- Accurate result
- Sensitve CMOS detection and precise automatic stage
- Auto focusing
- Cell therapy quality control
APPLICATIONS
- Stem cell
- CAR-T cell
- CAR-NK cell
- Adipose-derived stem cell
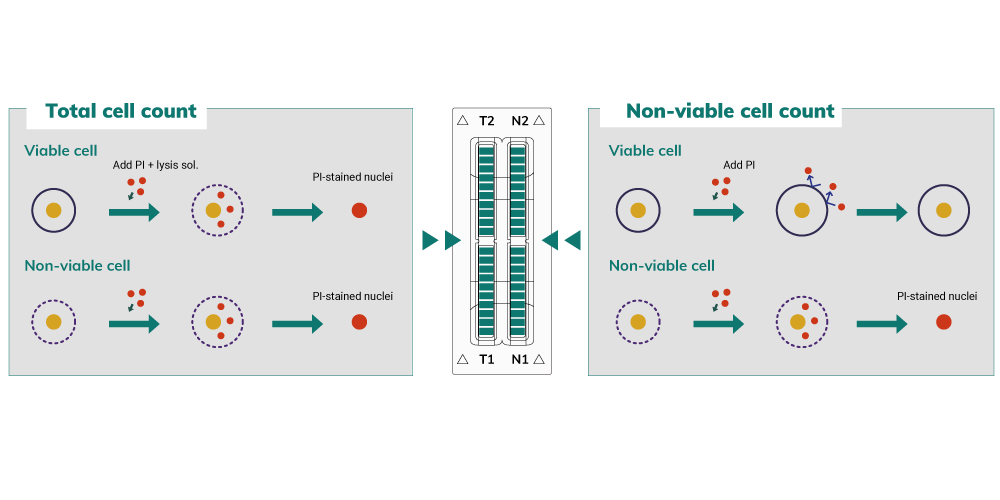
Principle of Viability Measurement (PI-Staining Method)
After the samples are stained with ˜uorescent dye, propidium iodide, which intercalates DNA to stain the nucleus of target cells, ADAM takes ˜uorescent images automatically. The obtained images are processed by image analysis software integrated inside the system.
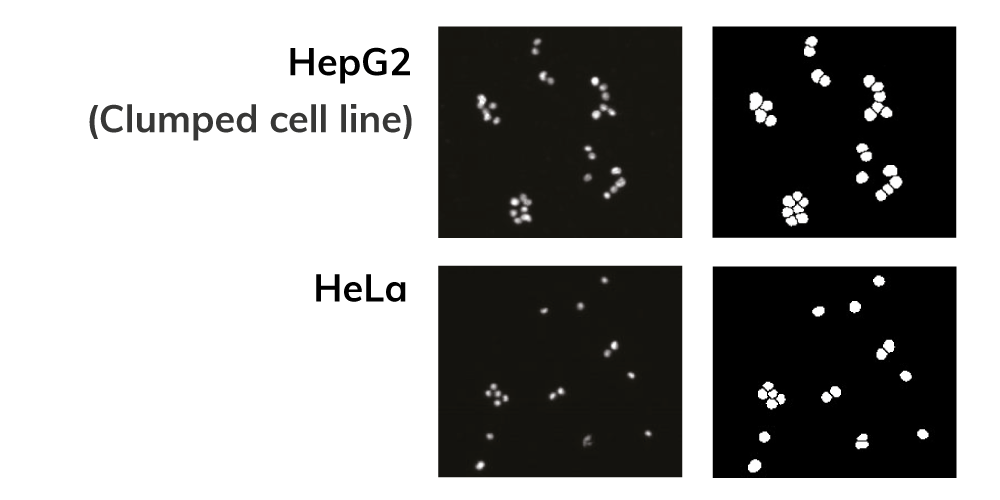
Counting Aggregated and Irregular-Shaped Cells
ADAM provides accurate and reliable results because it counts aggregated and irregular-shaped cells.
- Accurate count based on cell size and shape
- Count aggregated cells individually
- Debris is excluded from results
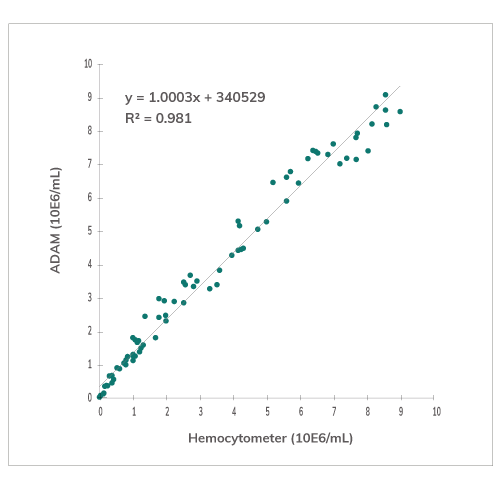
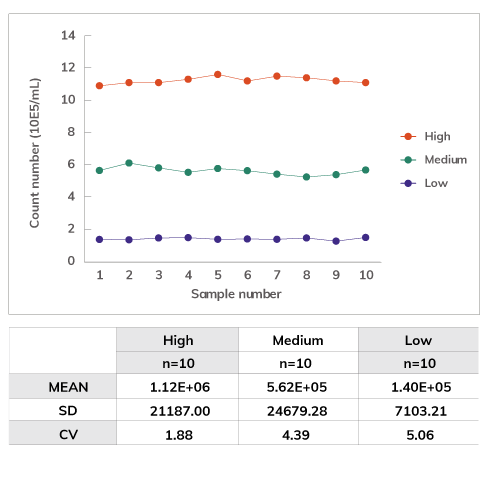
Accuracy & Repeatability
Correlation of total cell counting between hemocy-tometer and ADAM using SH-SY5Y cells.
Sample with low, medium and high concentration of cells were counted with ADAM. The repeatability at each level of cell concentration is high.
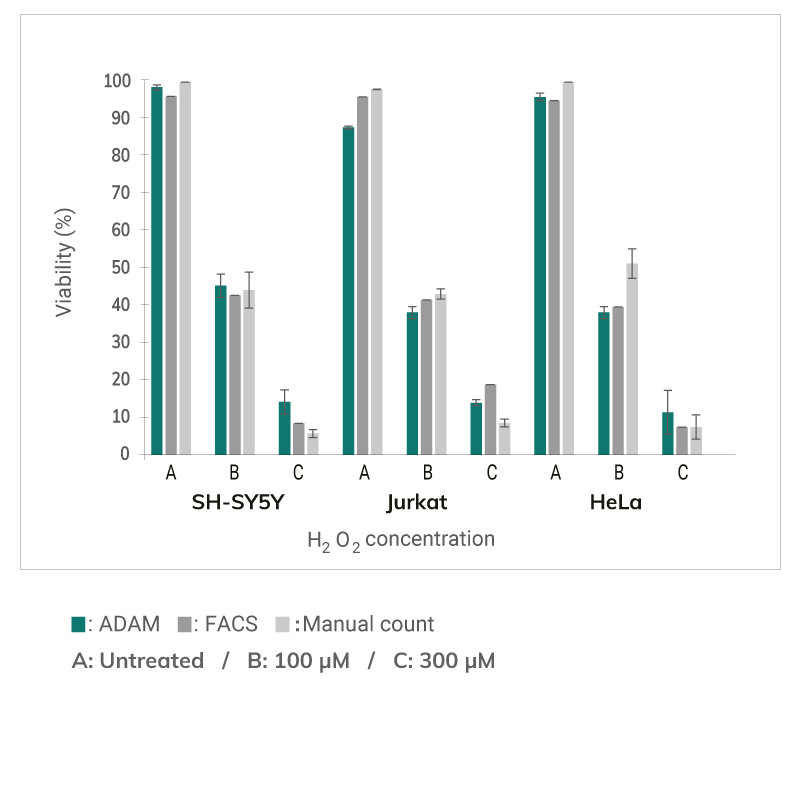
Comparison of Cell Viability
Comparison of cell viability between ADAM, flow cytometry, and manual counting.
SH-SY5Y, Jurkat, HeLa cells were treated with 100, 300μM H2O2 for 3 hours, then analyzed by ADAM, flow cytometry, and manual counting.
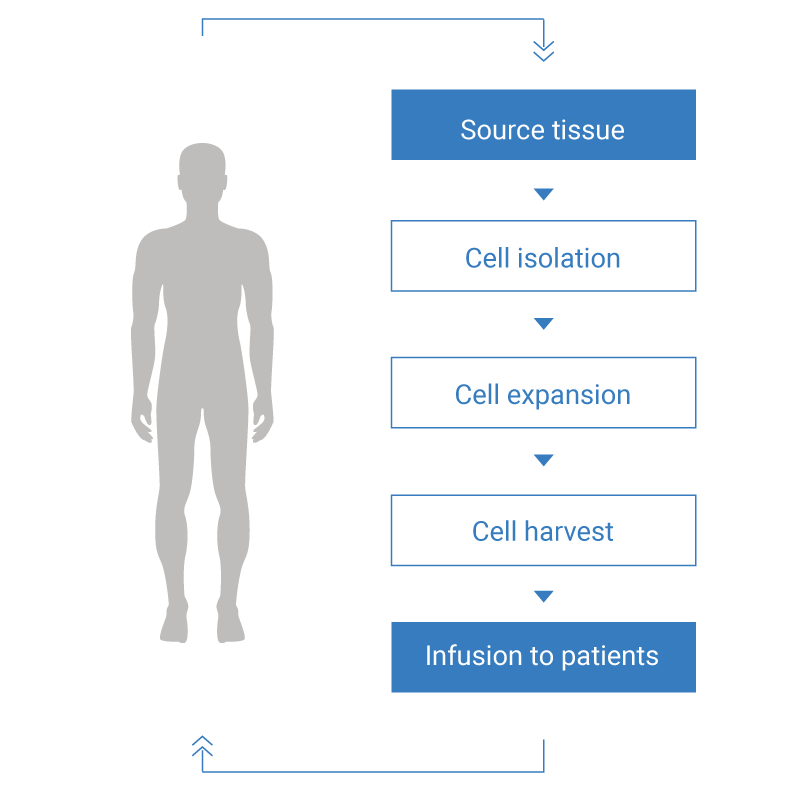
Cell Therapeutic Applications
ADAM can be used as a device for monitoring and QC of the cell numbers and viability in the process of manufacturing cells (CAR-T cells, stem cells, etc.) for Cell Therapy.
In addition, it is possible to use ADAM depending on the cell types (Whole blood cell, PBMCs, etc.) that needs to be monitored during the manufacturing of cell therapy products.
- Stem cell
- CAR-T cell
- CAR-NK cell
- Adipose-derived stem cell
- Whole blood cell
- Aggregated cell
- PBMCs
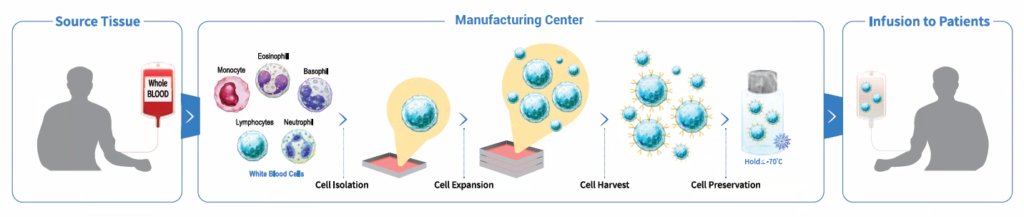
QC Platform for Producing CAR-T cell
It is easy to monitor all di°erent steps of the puriÿcation, expansion, and formulation of CAR-T cells using the ADAM to ensure precise and reliable results. ADAM can be used for cGMP, process control and quality control of CAR-T cell.
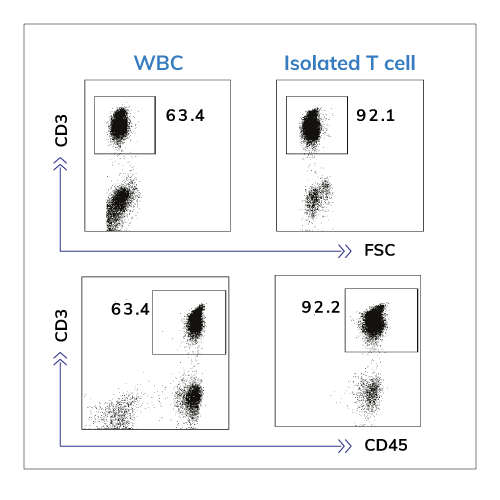
Performance Test from Isolated T Cells
The profiles of T cells which was used for performance evaluation – Flow cytometric analysis of CD3 expression on unsorted (WBC; left panel) or sorted (Isolated T cell; right panel) human peripheral blood lymphocytes.
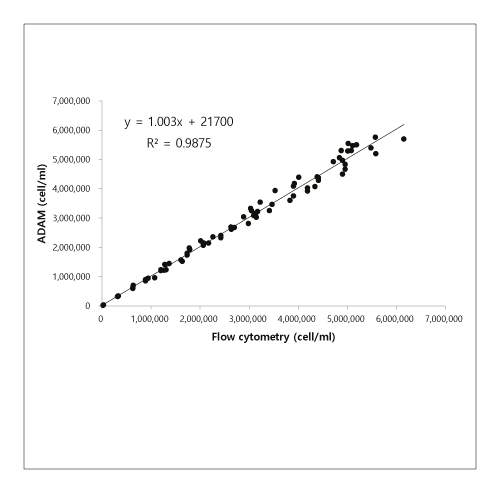
Accuracy
Correlation of T cell counting between flow cytometry and ADAM in Isolated T cells.
Method comparison between ADAM and flow cytometry.
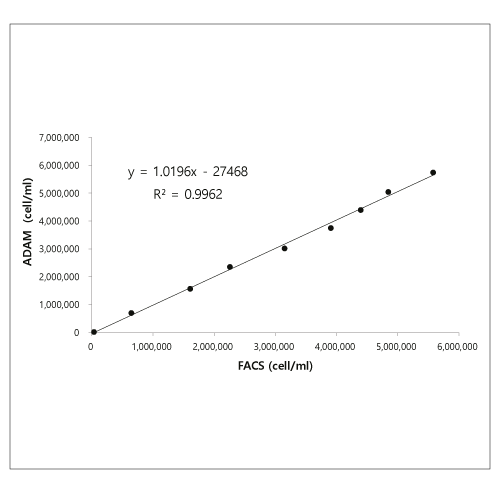
Linearity
Comparison between flow cytometry and ADAM in Isolated T cells.
Repeatability
The sample that low, medium, and high concentra-tion of isolated T cells were counted with ADAM.
| Low | Medium | High | |
| MEAN | 486,336 | 1,446,250 | 2,905,800 |
| CV | 4.97 | 3.05 | 1.65 |
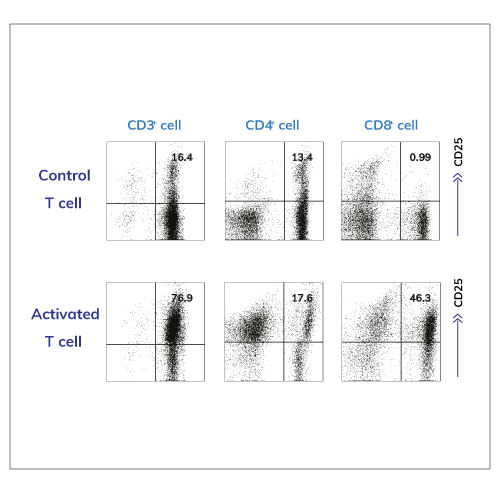
Performance Test from Activated T Cells
The phenotypes of activated T cells which was used for performance evaluation – Flow cytometric analysis of CD25 expression on TCR stimulated (Activated T cell; bottom panel) or unstimulated (control T cell; upper panel) T cells.
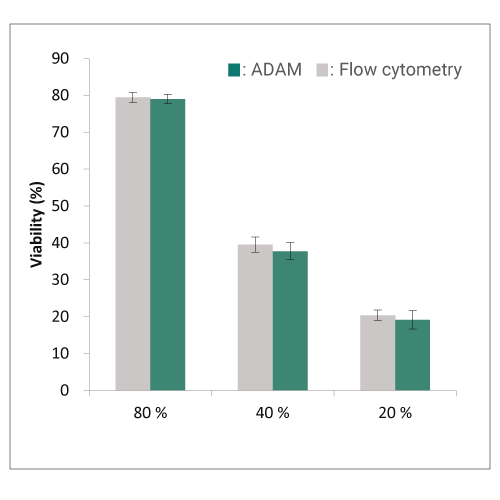
Viability
Comparison of viability between flow cytometry and ADAM-CellT in Activated T cells.
Repeatability
The sample that low, medium, and high concentration of activated T cells were counted with ADAM.
| Low | Medium | High | |
| MEAN | 514,470 | 1,436,925 | 2,887,715 |
| CV | 9.46 | 6.47 | 5.05 |
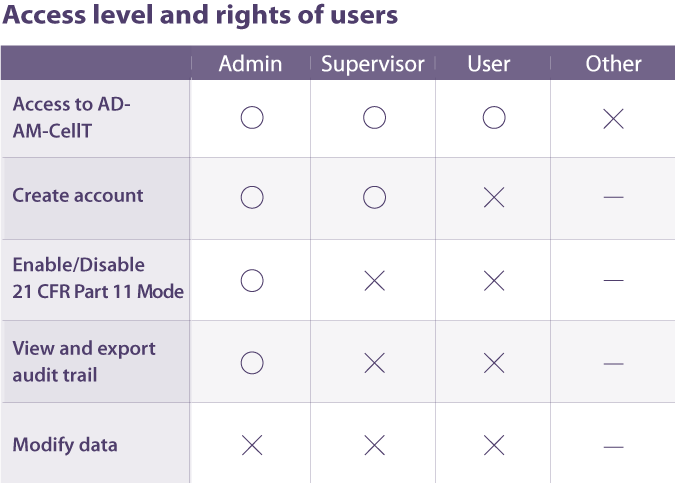
Data Management
ADAM-CellT complies 21 DFR part 11 which is a regulation about electronic records and signatures for use in cGMP facilities.
When 21 CFR Part 11 mode is activated, data cannot be modified by any user. Every action of users is recorded in an audit trail which includes the date, time, and specific details of the action.
Specifications
| Focus |
Auto-focusing |
| LED |
4W Green LED |
| Loading Volume |
23 μL (2ch), 13 μL (4ch) |
| Measurement range |
5 X 10E4 ~ 4 X 10E6 cells/mL |
| Measuring Volume |
8.6 μL (2ch), 3.4 μL (4ch) |
| Analysis time |
< 50 sec / test (2ch), < 25 sec / test (4ch) |
| Size (L x W x H) |
227x276x270mm |
| Weight |
7 kg |
Accessories
Size: 1 Kit | 200 slides
Staining method - Propidium Iodide (PI)